
Mass vaccination is an important key to prevent the spread and help bring an end to the novel coronavirus (COVID-19) pandemic. Currently, countries across the world are diligently working to ensure more vaccines reach those in need. In Japan, COVID-19 vaccination began in February 2021 and more than 200 million dozes has been administered to the population. (As of February, 2022).
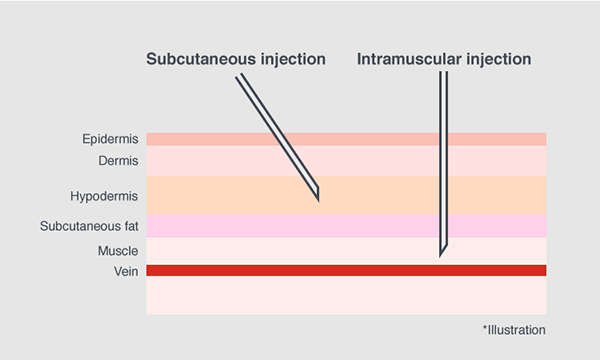
The COVID-19 vaccines used in Japan today are for intramuscular injection. Yet, subcutaneous injection is most major for vaccines in Japan. In order to swiftly launch the vaccination program there was a need to speed up production of injection devices that is suitable for the occasion.
Today, COVID-19 vaccinations continue to take place at an unprecedented scale and pace. We will introduce Terumo's contributions and commitment to vaccination accessibility, from the viewpoint of a manufacturer of injection syringes.
Timeline of Events
| January 15, 2020 | First COVID-19 case confirmed in Japan |
| April 7, 2020 | Japanese Government declares "State of Emergency" COVID-19 preventative measure (first time) |
| December 2020 | The first COVID-19 vaccine receives Emergency Use Authorization (EUA) in the US |
| January 2021 | Terumo starts developing a new low dead-volume (LDV) syringe to be used for COVID-19 vaccines |
| February 2021 | Vaccination starts in Japan |
| March 31, 2021 | Terumo starts production of new LDV syringes |
Accelerating the development of syringes for efficient vaccination
During the H1N1 virus pandemic in 2009, Terumo developed and launched the fixed-needle type LDV syringe which reduces the amount of liquid remaining inside the syringe — in order to make use of precious doses of vaccine without waste.
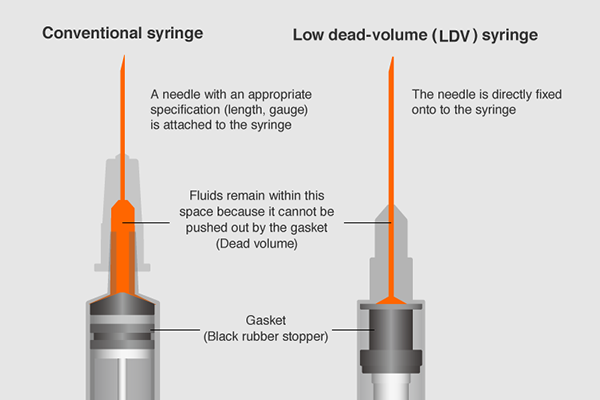
However, because this syringe is originally designed for subcutaneous injection, the needle was slightly too short for the COVID-19 vaccine which needs to be injected to the muscle.
The utmost priority was to develop a reliable syringe as quickly as possible so it was sensible to utilize its existing technology. Terumo employed the syringe design from the LDV syringe launched in 2009 as a starting point and redesigned the length of the needle to meet appropriate specification needs for the COVID-19 vaccines.
The country-wide COVID-19 vaccination scheme had another unprecedented element. A large indefinite number of healthcare professionals, including doctors and nurses from various backgrounds, will administer the vaccine. As such, engineers at Terumo conceptualized the need for a syringe that anyone can use easily and safely. After research of past scientific journals and conducting in-house experiments, they determined the appropriate needle length specially designed for the occasion.
The new LDV syringe was quicky approved by the regulatory authorities and was ready to be delivered to vaccination sites in just three months after starting development.
When designing the product, we had one goal in mind: to promptly provide an effective injection device that will support vaccination accessibility so that as many people as possible can receive the COVID-19 shots. I believe that we were able to accelerate the development process because all the related departments in Terumo worked in coordination as a team on every step of the process—from the product design, regulatory approval and production.
It was a very hectic few months at the time, but whenever I would see the syringes show up on the news, a sense of confidence grew and I was able to realize how much we are contributing to society. Syringes are products that are commonly used in medical settings today. However, I feel accomplished that we were able to improve and bring an important value to a seemingly “ordinary” product.

A stable supply to continue supporting the frontlines
As of February 2022, approximately 80 percent of the Japanese population has received the COVID-19 vaccine. To continue to meet the demand of many, it is important to efficiently and stably produce the product with consistent quality. Terumo plans to produce approximately 30 million syringes by March 2022, and are preparing to enlarge the facility so that production capacity can double up starting from the following fiscal year.
Terumo's Kofu Factory, where the LDV syringes are manufactured, uses integrated automatic production where most of the production processes are automated—from molding the plastic to assembling the parts, packing, and sterilization. Therefore, all the production facility is custom-made to fit Terumo's specifications. Currently, with the new facility being installed, associates at Terumo are carefully adjusting the facility to further increase production capacity.
We have formed a project team at Kofu Factory combining all our technology and production know-how. We are committed to providing LDV syringes to more people; and we are currently working hard every day to promptly set up the new facility through repeated trial and error. This is all in hopes to increase vaccination accessibility.
The new hybrid facility is equipped with the ability to produce multiple products in one production-line. Through our facilities and products, I aspire to achieve the true meaning of our Corporate Mission of "Contributing to Society through Healthcare" and help not only COVID-19 patients but also various patients who need medical care.


